Background: Recently, the first-line treatment of MCL has been continuously evolving with the introduction of targeted agents and chemo-free regimens. It is well-known that bruton's tyrosine kinase (BTK) inhibitors were effective with MCL patients (pts). Orelabrutinib (O), a novel highly selective BTK inhibitor, has shown high response rates and good tolerability in R/R MCL (Song et al. Blood. 2020). Besides, O combined with rituximab (R) could preserve NK cell mediated ADCC induced by R and enhance the apoptosis of tumor (Yu et al. Mol Ther Oncolytics. 2021).
Thus, we hypothesized that OLR would exhibit synergistic antitumor activity in MCL and improve the depth and durability of responses. Here, we reported the preliminary data on efficacy, safety, and mutation spectrum of OLR regimen in untreated MCL from the POLARIS study (NCT05076097).
Methods: Pts ≥18 years with untreated MCL were enrolled in this prospective, multicenter, single-arm phase 2 study. All pts received OLR-induction therapy on a 28-day cycle for 6 cycles (O, 150 mg/d; L, 15 mg on days 1-21, then 20 mg of cycles 2-6 if tolerated or 10 mg if not tolerated; R, 375 mg/m 2 on days 1, 8, 15, 22, then day 1 of cycles 3, 5), followed by OLR-maintenance therapy for up to 18 cycles. Primary endpoint was complete response rate (CRR) after 6 cycles of induction treatment. Secondary endpoints included overall response rate (ORR), time to response (TTR), duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety. Exploratory endpoint was to explore the prognostic value of detecting peripheral blood (PB) ctDNA by NGS.
Results: As of July 10, 2023,a total of 28 pts (male, 89.3%; median age, 60.0 y [range, 53.0-64.5]) were enrolled. Baseline characteristics included 96.4% Eastern Cooperative Oncology Group performance status of 0-1, 82.1% stage III-IV disease, 57.1% Ki67 index (≥30%), 75.0% low-risk MIPI scores, 42.9% bone marrow involvement, and 60.7% maximum lesion diameter of ≥5 cm.
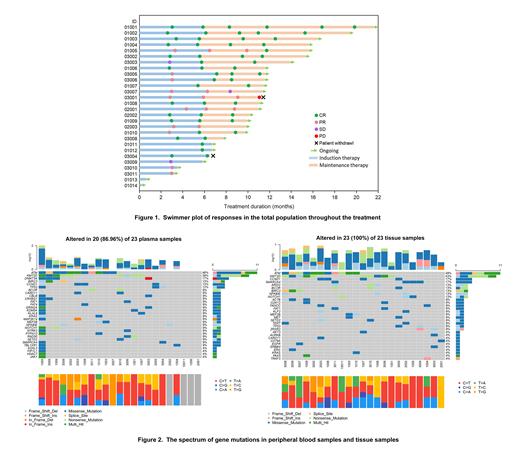
Twenty-one pts (75.0%) who have completed 6 cycles of induction therapy were evaluable for response, 16 (76.2%) had a complete response (CR) and 5 (23.8%) had a partial response (PR), with an ORR of 100% ( Figure 1). In addition, 18 of 21 pts were available for minimal residual disease (MRD) analysis, and PB-MRD and bone marrow MRD (BM-MRD) of the 18 pts were negative. The results of CRR were reported across different subgroups stratified by Ki67 index (<30% vs. ≥30%, 66.7% and 83.3%), MIPI scores (low vs. intermediate, 81.3% and 60.0%), maximum lesion diameter (<5 cm vs. ≥5 cm, 87.5% and 69.2%), and bone marrow involvement (yes vs. no, 83.3% and 73.3%). With a median follow-up of 11.0 months, the median TTR was 3.0 months (range, 2.8-3.4). Median DOR and median PFS were not reached, with the estimated 12-month DOR rate and PFS rate of 90.9% and 92.3%, respectively. Mutationalspectrum (475 panels) was determined in 23 pts. The spectrum of gene mutations is depicted by PB samples and tissue samples at baseline in Figure 2. The most common mutated genes in tissues samples were: ATM (48%), KMT2D (43%), CCND1 (17%), SMARCA4(17%), ARID2 (13%), BCOR (13%), BIRC3 (13%), NFKBIE (13%), NOTCH1 (13%), ACTB (9%), CDK12 (9%), FANCC(9%),FAT1(9%),KLF2(9%),MEF2B(9%),MET(9%),SETD2(9%), TP53 (13%),TERT(9%). The most common mutated genes in PB samples were: ATM (48%), KMT2D (39%), DNMT3A (17%), BIRC3 (13%), CCND1 (13%), TP53 (13%), BCOR (9%), CARD11 (9%), CBLB (9%), CRYBG1 (9%), FAT1(9%), NOTCH1 (9%), GRIN2A (9%), KDM5A (9%), KLHL6(9%), KRAS (9%), MAP3K14 (9%). For all gene mutations, 75.4% (43/57) detected in tissue were also found in PB samples. Further, more additional mutated genes can be detected in plasma. Meanwhile, 4 of 23 pts had a PR, 1 of 4 had multiple gene mutations in PB samples, including BTK and TP53. This suggests that ctDNA detection may potentially predict that these gene mutations are associated with depth of remission.
Twenty-six (92.9%) pts experienced adverse events (AEs). The most common AEs (any grades, ≥3 grades) were neutropenia (64.3%, 39.3%), leukopenia (60.7%, 14.3%), thrombocytopenia (35.7%, 7.1%), lymphopenia (35.7%, 3.6%), and COVID-19 infection (28.6%, 7.1%). At the time of data cut-off, 26 pts remained on study. No deaths were observed.
Conclusions: The preliminary findings show that the OLR exerted synergistic antitumor activity, with manageable toxicity in untreated MCL. Detection of PB ctDNA by NGS method may help to predict prognosis.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal